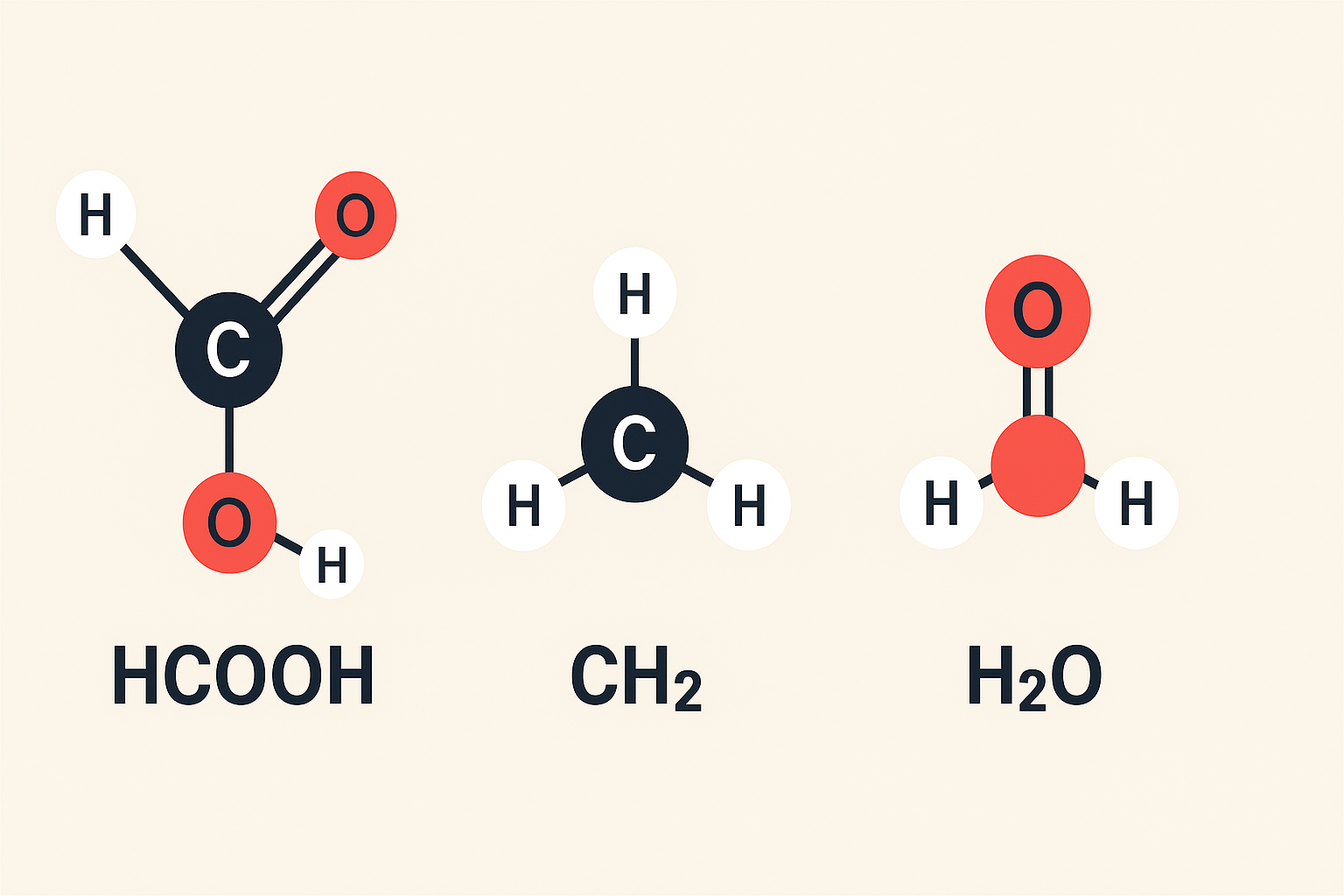
What “HCOOH CH2 H2O” Might Mean in Simple Words
The string HCOOH CH2 H2O looks like three pieces set side by side: HCOOH, CH2, and H2O. HCOOH is formic acid, a small organic acid found in ants and used in many industries. CH2 is a two-hydrogen, one-carbon fragment that shows up inside bigger molecules. H2O is plain water. When written in one line, HCOOH CH2 H2O is not a standard full formula. Think of it as three clues. In this guide we explain each clue, how they connect, and what reactions or mixtures you might see in schoolwork, labs, or industry.
A Quick Primer on the Three Parts You See
Start with HCOOH. It is the simplest carboxylic acid, also called formic acid. Next is CH2, a building block chemists use when drawing longer chains like CH3–CH2–OH. Last is H2O, which is water, a solvent, a reactant, and the world’s most common liquid. When someone writes HCOOH CH2 H2O, they may be asking about mixing formic acid with water, or about a reaction that inserts a CH2 group into a chain related to formic acid. The best way to understand it is to break the puzzle into the roles each piece plays.
Understanding HCOOH (Formic Acid) the Easy Way
HCOOH has one carbon, two oxygens, and two hydrogens. Its shape includes a carbonyl group (C=O) and an –OH group on the same carbon, which makes it a carboxylic acid. Because it is small, it is quite acidic compared with longer acids. In water, HCOOH can donate a proton (H⁺), so solutions of HCOOH are acidic. People use it to tan leather, preserve animal feed, adjust pH in processes, and as a cleaner in some controlled settings. When you see HCOOH CH2 H2O, remember that the HCOOH part brings acidity and a carbon backbone that can react in many beginner-friendly organic pathways.
What CH2 Means and Why It Looks Unfinished
The CH2 piece by itself is not a full stable molecule in a bottle. It is more like a unit within a bigger molecule or a short-hand in an equation. For example, propane can be written as CH3–CH2–CH3. You also see CH2 in repeating units like a polymer chain, or in drawings where chemists show only the changing part of a structure. So when you meet HCOOH CH2 H2O, the CH2 may hint at a chain extension, a side group near formic acid, or a generic placeholder in a reaction scheme. It is a clue that carbon chemistry is involved rather than a finished compound.
Water’s Role (H2O) as Solvent and Reactant
H2O is more than a backdrop. It dissolves many acids, including HCOOH, and it can join reactions through hydrolysis or hydration. In a beaker, HCOOH CH2 H2O often means you are dealing with an aqueous solution of formic acid and some organic fragment that includes CH2. Water’s polarity helps ions move. It also affects reaction rates and equilibrium. In simple classroom terms, water can make a reaction go faster, slower, or cleaner. That is why scientists always note if a reaction is “in water,” “in alcohol,” or “neat.” The H2O part of HCOOH CH2 H2O tells you the stage where chemistry happens.
How HCOOH Behaves in H2O and Why pH Matters
Put HCOOH in H2O and you get an acidic solution. The acid partially donates H⁺ to water, forming hydronium ions. The amount depends on concentration and temperature. For a young learner, think of acid strength like the tang in lemonade: more acid, more tart. Formic acid is stronger than acetic acid (vinegar), so solutions can be more biting. When anyone writes HCOOH CH2 H2O, they could be setting up a scenario where pH control matters. In many reactions that involve a CH2 fragment, the right pH keeps side reactions away, improves yields, and protects equipment.
Possible Meanings in Reactions and Mechanisms
In simple organic lessons, HCOOH can be a reagent, a source of hydrogen in reductions, or a temporary “helper” that adjusts conditions. The CH2 clue could refer to a methylene group being added, removed, or shifted in a pathway. With H2O present, you may see hydration, hydrolysis, or just a good medium for heat transfer. So HCOOH CH2 H2O might label a reaction mixture where formic acid and water help transform a compound that has a CH2 unit, or where the product gains or loses a CH2 in a gentle, water-tolerant process. Teachers often compress these hints into short strings.
Real-World Uses Where This Trio Makes Sense
Industry uses HCOOH in water to control pH during cleaning or processing. Farmers use formic acid solutions in feed preservation. Labs may use HCOOH/H2O mixes in chromatography or sample prep. The CH2 part shows up inside fuels, plastics, fragrances, and everyday solvents. You may not see a bottle marked HCOOH CH2 H2O, yet the idea of formic acid in water acting on or with molecules that contain CH2 is common. In short, the string is a shortcut that points to practical setups: an acidified water system working with organic materials that include CH2 units.
Safety Basics for Handling HCOOH in Water
Formic acid can irritate skin, eyes, and airways. When mixing HCOOH with H2O, always add acid to water slowly, not water to acid. Wear gloves, goggles, and a lab coat. Work in a ventilated space. Label containers clearly, never in drink bottles. If you spill, dilute with lots of water and follow local rules for cleanup. Respect the acid, even if it seems simple. If your work involves compounds with CH2 groups, remember that some organics are flammable, so keep away from sparks or flames. These rules apply whether or not someone writes the setup as HCOOH CH2 H2O.
Easy Lab Tips for Beginner Success
Plan before you pour. Measure your HCOOH volume, record your H2O volume, and write down your target pH. Stir well and let temperature settle because acids can warm solutions. If a CH2-containing reagent is part of the plan, check its solubility in water first. If it floats or separates, you may need a co-solvent or an emulsifier. Keep notes with times, temperatures, and observations like color or smell. When you see HCOOH CH2 H2O in a task list, think: safe mixing, good stirring, pH check, and careful logging. Simple habits make results repeatable and trustworthy.
Key Takeaways You Can Trust
Think of HCOOH CH2 H2O as a compact message. HCOOH stands for formic acid, a useful, small carboxylic acid. CH2 points to carbon chain parts inside many organic molecules. H2O is water, the classic solvent and sometimes a reactant. Together, they signal an acidic, water-based setup acting on or with organic material that contains CH2. This shows up in labs, teaching demos, and industry. It is not a bottle label, but it is a helpful index card for a planned mixture or reaction. Use safe handling, check pH, and document steps for reliable, repeatable outcomes.
Where You See It in Study and Work
In school, you might see HCOOH CH2 H2O in a practice problem about acid strength, buffer planning, or a mild organic reaction. In labs, it could appear in a method for cleaning glassware, conditioning a column, or adjusting a mobile phase. In factories, it might be part of a standard operating procedure for a wash, a feed preservative, or a gentle surface prep. Even if the string is shorthand, the parts are real. If you can explain each part of HCOOH CH2 H2O to a friend, you are already thinking like a chemist who values clarity and safety.
How to Explain It to a Younger Learner
Use a kitchen model. Say HCOOH is a strong lemon taste, CH2 is a single block you use to build long toy trains, and H2O is the bowl where you mix things. When you put them together, you are not making a new special toy, but you create the right place and flavor for building. That is what HCOOH CH2 H2O means. It is a sign that the bowl is filled with water, the taste is acid, and the building blocks include a CH2 piece. This simple story helps kids remember what the letters stand for in a calm, friendly way.
Simple Troubleshooting When Results Look Off
If a mix labeled HCOOH CH2 H2O does not behave as expected, check five things. First, confirm your concentrations. Second, verify pH with fresh strips or a meter. Third, look at temperature because reactions can be sensitive to heat. Fourth, inspect solubility. If the CH2-rich material will not dissolve, consider a co-solvent. Fifth, review time. Some steps need minutes, others need hours. Write down changes one by one, not all at once, so you learn which factor fixed the issue. Slow, careful changes move you from confusion to control without wasting material.

1: Is This a Valid Single Chemical?
No. HCOOH CH2 H2O is not a single stand-alone compound you can buy. It reads like three items placed together. Still, it is useful shorthand. It tells you formic acid is present, water is the medium, and a CH2 fragment or chain is in play. In textbooks and notes, people compress lines to save space. That is fine if everyone understands the parts. If you need a real chemical name, say “formic acid in water” and then name the actual compound that carries the CH2 group. Clarity beats speed. Use the full names when you order or label.
2: Can I Mix These at Home or School?
Only with proper training and supervision. Formic acid can harm skin and eyes. Even small spills can sting. If your teacher assigns work that looks like HCOOH CH2 H2O, they will provide safe quantities, protective gear, and instructions. At home, do not experiment with acids without adult guidance and safety supplies. For young learners, a safe way to explore acidity is with kitchen acids like vinegar and baking soda under supervision. Save HCOOH CH2 H2O style experiments for a real lab where you have sinks, eyewash, and a teacher who shows the correct steps.
3: What Happens if Water Is Not Used?
Without H2O, your system may react slower, faster, or not at all, depending on the chemistry. Water dissolves ions and can stabilize charged steps in mechanisms. If you swap H2O for alcohol or another solvent, acidity and solubility change. The same HCOOH amount may feel stronger or weaker. If your CH2-bearing reagent does not like water, a mixed solvent might work better. This is why short strings like HCOOH CH2 H2O are just starting points. The best approach is to test small batches, log your data, and adjust the solvent to match your target reaction or separation.
4: Why Use Formic Acid Instead of Another Acid?
HCOOH is small, effective, and familiar. It often gives cleaner control than strong mineral acids in gentle organic work. In water, it can set a useful pH range without adding heavy metals or halides. For systems involving a CH2 unit, that can matter because harsh acids may cause side reactions like dehydration or polymerization. Formic acid is also common in analytical workflows. That said, it is not the only choice. Acetic acid, citric acid, or dilute mineral acids may work too. If someone writes HCOOH CH2 H2O, they likely want the balance of strength and simplicity formic acid brings.
Conclusion and Strong Call to Action
Now you can read HCOOH CH2 H2O with confidence. It is a compact way to say formic acid, a CH2-bearing organic context, and water as the medium. You learned what each part does, how pH guides outcomes, and how to stay safe and organized. If you need a step-by-step plan for your specific lab or a classroom worksheet that uses HCOOH CH2 H2O in practice problems, tell me your goal and grade level. I will tailor a clean, ready-to-use guide with exact quantities, pH targets, and safety notes so you can run it smoothly on day one.
You May Also Like: Understanding Hizzaboloufazic: Uses, Benefits, and Concerns